Introduction
With 28 years of multi-source manufacturing expertise, Runxin presents Chondroitin Sulfate 90% USP40 Solutions—comprehensive strategies for pharmaceutical and nutraceutical applications requiring strict USP40 compliance. This guide addresses formulation challenges while providing certified solutions for quality assurance and regulatory success.
Technical Specifications & USP40 Compliance
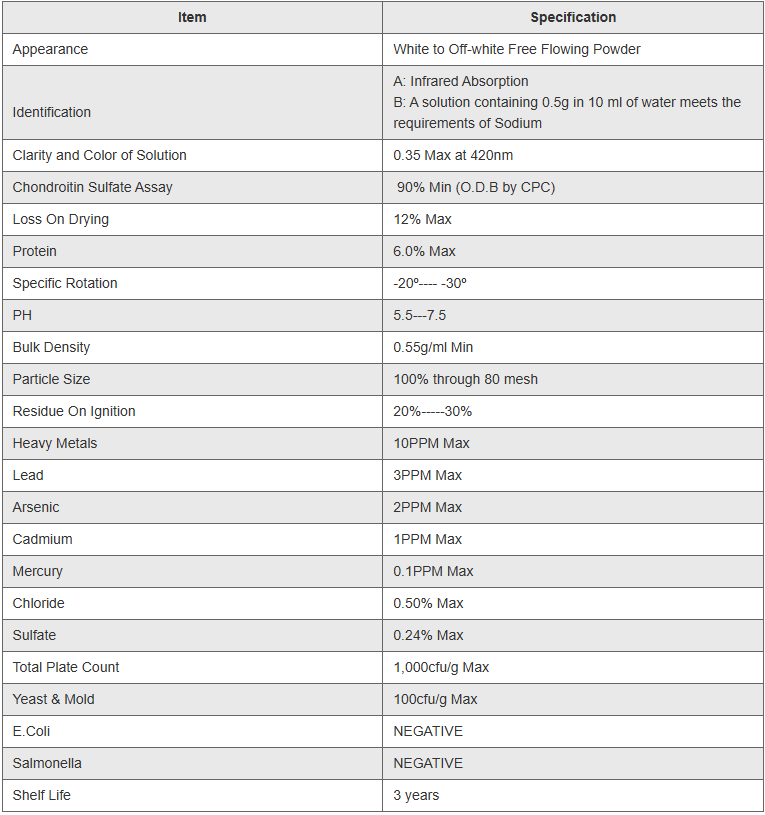
Guaranteed 90% minimum purity per USP40 standards
Multi-source availability (bovine, porcine, marine, avian)
Full USP40 testing methodology compliance
Complete traceability and source documentation
![37 硫酸软骨素90%参数 37 硫酸软骨素90%参数]()
Professional Application Solutions
1. USP40 Compliance Solutions
2. Multi-Source Integration Solutions
3. Global Regulatory Solutions
4. Quality Assurance Solutions
Certification Excellence & Quality Systems
Complete Certification: cGMP, ISO9001, ISO22000, ISO13485, HACCP, HALAL, FSSC22000
USP40 Compliance: Full testing methodology implementation
Quality Verification: Regular third-party testing validation
Documentation Support: Complete regulatory and compliance packages
5 Google-Optimized Q&A About Chondroitin Sulfate 90% USP40
Q1: What does USP40 certification mean for chondroitin sulfate?
A: USP40 certification guarantees the product meets the latest United States Pharmacopeia standards for identity, purity, strength, and quality, including specific testing methodologies and 90% minimum purity requirements.
Q2: How does multi-source manufacturing benefit customers?
A: Multi-source capability provides supply chain flexibility, allows source selection based on regional regulations, and ensures continuous availability regardless of individual source shortages.
Q3: What documentation verifies USP40 compliance?
A: Runxin provides USP40 Certificate of Analysis, Method Validation Reports, and complete testing documentation demonstrating compliance with all USP40 requirements.
Q4: How do you maintain consistent 90% purity across different sources?
A: Through standardized purification processes, rigorous quality controls, and continuous process validation under our cGMP and ISO9001 certified quality systems.
Q5: What solutions support international regulatory compliance?
A: We offer source-specific documentation, regional compliance certificates, and technical support tailored to meet the requirements of different international markets.
Application Solutions
Pharmaceutical Formulations: USP40 compliant drug products
Dietary Supplements: High-quality nutraceutical products
International Exports: Region-specific compliant products
Quality-Critical Applications: Products requiring verified USP40 compliance
Regulatory Submissions: Documentation-ready material for approvals
Technical Support Services
USP40 compliance verification
Source selection consultation
Regulatory documentation preparation
Quality assurance protocol development
Conclusion
Runxin's Chondroitin Sulfate 90% USP40 Solutions deliver guaranteed quality and regulatory compliance for critical applications, supported by multi-source manufacturing flexibility and 28 years of pharmaceutical ingredient expertise.
Contact Runxin for USP40 solutions, multi-source options, and comprehensive technical support.
![CS CS]()